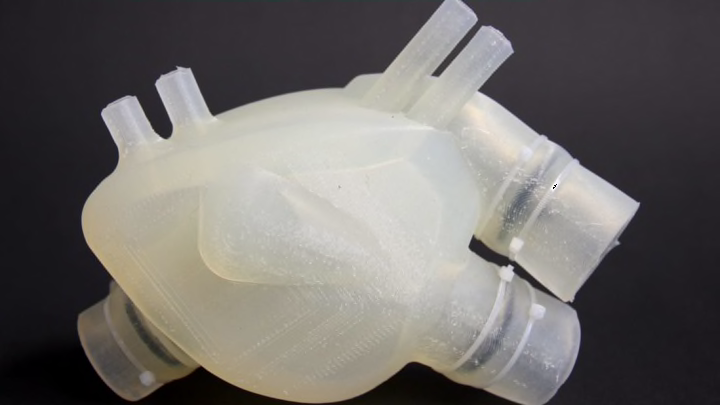
If the heart in the Functional Materials Laboratory at ETH Zurich University were in a patient in an operating room, its vital signs would not be good. In fact, it would be in heart failure. Thankfully, it's not in a patient—and it's not even real. This heart is made of silicone.
Suspended in a metal frame and connected by tubes to trays of water standing in for blood, the silicone heart pumps water at a beat per second—a serious athlete's resting heart rate—in an approximation of the circulatory system. One valve is leaking, dripping onto the grate below, and the water bins are jerry-rigged with duct tape. If left to finish out its life to the final heartbeat, it would last for about 3000 beats before it ruptured. That's about 30 minutes—not long enough to finish an episode of Grey's Anatomy.
Nicolas Cohrs, a bioengineering Ph.D. student from the university, admits that the artificial heart is usually in better shape. The one he holds in his hands—identical to the first—feels like taut but pliable muscle, and is intact and dry. He'd hoped to demonstrate a new and improved version of the heart, but that one is temporarily lost, likely hiding in a box somewhere at the airport in Tallinn, Estonia, where the researchers recently attended a symposium.
Taking place over the past three years, the experimental research is a part of Zurich Heart, a project involving 17 researchers from multiple institutions, including ETH, the University of Zurich, University Hospital of Zurich, and the German Heart Institute in Berlin, which has the largest artificial heart program in Europe.
A BRIDGE TO TRANSPLANT—OR TO DEATH
Heart failure occurs when the heart cannot pump enough blood and oxygen to support the organs; common causes are coronary heart disease, high blood pressure, and diabetes. It's a global pandemic, threatening 26 million people worldwide every year. More than a quarter of them are in the U.S. alone, and the numbers are rising.
It's a life-threatening disease, but depending on the severity of the condition at the time of diagnosis, it's not necessarily an immediate death sentence. About half of the people in the U.S. diagnosed with the disease die within five years. Right now in the U.S., there are nearly 4000 people on the national heart transplant list, but they're a select few; it's estimated that upwards of 100,000 people need a new heart. Worldwide, demand for a new heart greatly outpaces supply, and many people die waiting for one.
That's why Cohrs, co-researcher Anastasios Petrou, and their colleagues are attempting to create an artificial heart modeled after each patient's own heart that would, ideally, last for the rest of a person's life.
Mechanical assistance devices for failing hearts exist, but they have serious limitations. Doctors treating heart failure have two options: a pump placed next to the heart, generally on the left side, that pumps the blood for the heart (what's known as a left ventricular assist device, or LVAD), or a total artificial heart (TAH). There have been a few total artificial hearts over the years, and at least four others are in development right now in Europe and the U.S. But only one currently has FDA approval and CE marking (allowing its use in European Union countries): the SynCardia total artificial heart. It debuted in the early '90s, and since has been implanted in nearly 1600 people worldwide.
While all implants come with side effects, especially when the immune system grows hostile toward a foreign object in the body, a common problem with existing total artificial hearts is that they're composed of hard materials, which can cause blood to clot. Such clots can lead to thrombosis and strokes, so anyone with an artificial heart has to take anticoagulants. In fact, Cohrs tells Mental Floss, patients with some sort of artificial heart implant—either a LVAD or a TAH—die more frequently from a stroke or an infection than they do from the heart condition that led to the implant. Neurological damage and equipment breakdown are risky side effects as well.
These complications mean that total artificial hearts are "bridges"—either to a new heart, or to death. They're designed to extend the life of a critically ill patient long enough to get on (or to the top of) the heart transplant list, or, if they're not a candidate for transplant, to make the last few years of a person's life more functional. A Turkish patient currently holds the record for the longest time living with a SynCardia artificial heart: The implant has been in his chest for five years. Most TAH patients live at least one year, but survival rates drop off after that.
The ETH team set out to make an artificial heart that would be not a bridge, but a true replacement. "When we heard about these problems, we thought about how we can make an artificial heart that doesn't have side effects," he recalls.
USING AN ANCIENT TECHNIQUE TO MAKE A MODERN MARVEL
Using common computer assisted design (CAD) software, they designed an ersatz organ composed of soft material that hews closely to the composition, form, and function of the human heart. "Our working hypothesis is that when you have such a device which mimics the human heart in function and form, you will have less side effects," Cohrs says.
To create a heart, "we take a CT scan of a patient, then put it into a computer file and design the artificial heart around it in close resemblance to the patient's heart, so it always fits inside [the body]," Cohrs says.
But though it's modeled on a patient's heart and looks eerily like one, it's not identical to the real organ. For one thing, it can't move on its own, so the team had to make some modifications. They omitted the upper chambers, called atria, which collect and store blood, but included the lower chambers, called ventricles, which pump blood. In a real heart, the left and right sides are separated by the septum. Here, the team replaced the septum with an expansion chamber that is inflated and deflated with pressurized air. This action mimics heart muscle contractions that push blood from the heart.
The next step was to 3D-print a negative mold of the heart in ABS, a thermoplastic commonly used in 3D printing. It takes about 40 hours on the older-model 3D printers they have in the lab. They then filled this mold with the "heart" material—initially silicone—and let it cure for 36 hours, first at room temperature and then in an oven kept at a low temperature (about 150°F). The next day, they bathed it in a solvent of acetone, which dissolved the mold but left the printed heart alone. This process is essentially lost-wax casting, a technique used virtually unchanged for the past 4000 years to make metal objects, especially bronze. It takes about four days.
The resulting soft heart weighs about 13 ounces—about one-third more than an average adult heart (about 10 ounces). If implanted in a body, it would be sutured to the valves, arteries, and veins that bring blood through the body. Like existing ventricular assist devices and total artificial hearts on the market, it would be powered by a portable pneumatic driver worn externally by the patient.
FROM 3000 TO 1 MILLION HEARTBEATS
In April 2016, they did a feasibility test to see if their silicone organ could pump blood like a real heart. First they incorporated state-of-the-art artificial valves used every day in heart surgeries around the world. These would direct the flow of blood. Then, collaborating with a team of mechanical engineers from ETH, they placed the heart in a hybrid mock circulation machine, which measures and simulates the human cardiovascular system. "You can really measure the relevant data without having to put your heart into an animal," says Cohrs.
Here's what the test looked like.
"Our results were very nice," Cohrs says. "When you look at the pressure waveform in the aorta, it really looked like the pressure waveform from the human heart, so that blood flow is very comparable to the blood flow from a real human heart."
Their results were published earlier this year in the journal Artificial Organs.
But less promising was the number of heartbeats the heart lasted before rupturing under stress. (On repeated tests, the heart always ruptured in the same place: a weak point between the expansion chamber and the left ventricle where the membrane was apparently too thin.) With the average human heart beating 2.5 billion times in a lifetime, 3000 heartbeats wouldn't get a patient far.
But they're making progress. Since then, they've switched the heart material from silicone to a high-tech polymer. The latest version of the heart—one of which was stuck in that box in the Tallinn airport—lasts for 1 million heartbeats. That's an exponential increase from 3000—but it's still only about 10 days' worth of life.
Right now, the heart costs around $400 USD to produce, "but when you want to do it under conditions where you can manufacture a device where it can be implanted into a body, it will be much more expensive," Cohrs says.
The researchers know they're far from having produced an implantable TAH; this soft heart represents a new concept for future artificial heart development that could one day lead to transplant centers using widely available, easy-to-use design software and commercially available 3D-printers to create a personalized heart for each patient. This kind of artificial heart would be not a bridge to transplantation or, in a few short years, death, but one that would take a person through many years of life.
"My personal goal is to have an artificial heart where you don't have side effects and you don't have any heart problems anymore, so it would last pretty much forever," Cohrs says. Well, perhaps not forever: "An artificial heart valve last 15 years at the moment. Maybe something like that."